Few people realize that upon leaving the dentist’s office, their new filling, implant, or other dental restoration is already under attack by millions of oral bacteria.
Soon, the bacteria form biofilms, where they metabolize sugars and other carbohydrates into acids that can dissolve tooth structures and cause cracks—half of all restorations fail within 10 years—or lead to new decay and cavities called “secondary caries.”
Replacing restorations that fail due to cracking and secondary caries accounts for about 60 percent of all dental restorations performed in the U.S., at a cost of over $5 billion each year.
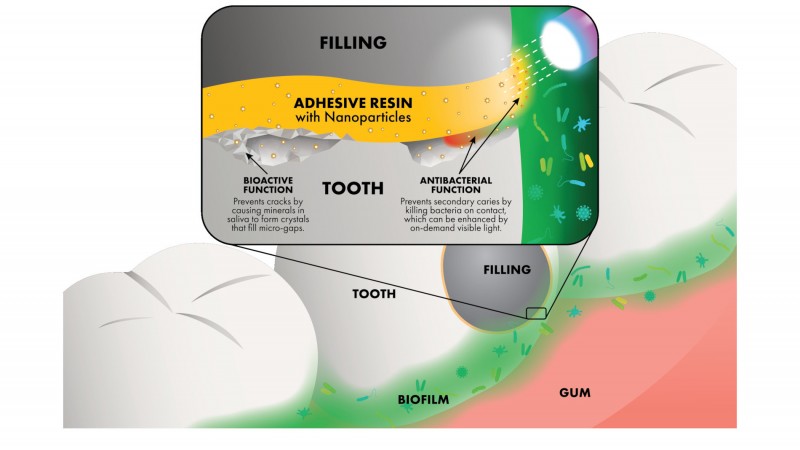
To help address the problem, Oak Ridge National Laboratory researchers are using neutron scattering to study how nanoparticles with antibacterial properties can be added to adhesive resins, which are used by dentists to strengthen the bond between a tooth and its polymer composite filling.
“The adhesive layer applied by a dentist prior to filling a cavity is fundamental to the success of the restoration, because the polymer materials used in fillings can promote the growth of biofilms,” said Fernando Luis Esteban Florez of the University of Oklahoma Health Sciences Center. “Also, tiny imperfections in the adhesive surface can lead to early-stage cracking that also contributes to the failure of restorations.”
Ideally, said Esteban Florez, an adhesive resin would have antibacterial properties and support the growth of dentin—the soft layer beneath a tooth’s hard enamel surface—to help eliminate small gaps in the adhesive layer.
Working with Adam Rondinone, a scientist at ORNL’s Center for Nanophase Materials Sciences, the researchers developed an experimental dental adhesive resin containing modified nanoscale titanium dioxide (N_TiO2) particles. They studied samples of the adhesive resin using small-angle neutron scattering at ORNL’s High Flux Isotope Reactor to determine the optimal shape, modifications, and dispersion for the particles.
“In creating the adhesive resin, we modified the surface of the N_TiO2 nanoparticles with silanes and proteins, to improve both the function of the nanoparticles within the polymer matrix and the ability of these materials to establish covalent bonds to a tooth’s naturally occurring proteins,” said Rondinone.
“The benefit of using the Bio-SANS beamline instrument at HFIR is that the neutrons can tell us how the proteins bond to the N_TiO2 and how the particles disperse.”
Early results show the nanoparticles disperse well and are compatible with the adhesive resin, a commercially available brand commonly used by dentists.
Other experiments have shown that the new adhesive resin exhibits active, on-demand antibacterial activity when irradiated by visible light, and passive, on-contact antibacterial effects even in darkness. Such a dual capability could enable a dentist to use light to jump-start the adhesive’s antimicrobial activity before filling the cavity, and afterward the adhesive would serve as a long-term, contact-based antibacterial barrier.
One of the next steps for the researchers is to use neutron scattering to evaluate the nanoparticles for potential bioactivity, including the ability to promote self-assembly of natural tooth material adjacent to the restoration.
“Studies have shown that nanoparticles can initiate the growth of crystalline structures and guide them to become chemically bound to teeth,” said Esteban Florez. “We plan to functionalize our N_TiO2 particles to produce crystals of hydroxyapatite, the primary component of dentin, which could promote the growth of the dentin layer to minimize gaps at the adhesive interface.”
He added, “Thanks to the user program at ORNL, even someone like me, a dentist with limited training in advanced scientific technologies, can test a hypothesis with the help of some of the top scientists in the field, while using world-class neutron scattering facilities. Together, hopefully, we can soon offer dentists a new and better tool that will give their patients longer lasting smiles.”
The work was supported by DOE’s Office of Basic Energy Sciences and the Oklahoma Center for the Advancement of Science and Technology.
The Center for Nanophase Materials Sciences and the High Flux Isotope Reactor at ORNL are DOE User Facilities.UT-Battelle manages ORNL for the DOE’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit http://science.energy.gov/.—by Paul Boisvert