Noncovalent Interactions Revealed by Neutron Diffraction
Scientific Achievement
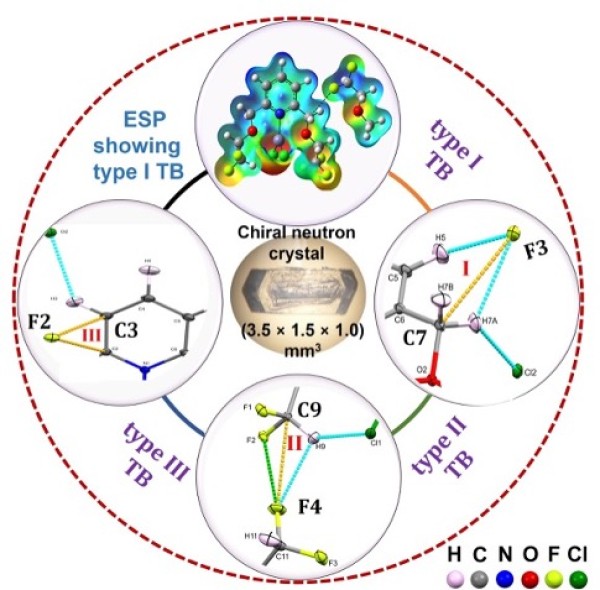
Determination of accurate hydrogen positions confirm unusual C−H bond elongations and shortenings in chiral zinc complexes predicted by density functional theory (DFT) calculations
Significance and Impact
Fundamental understanding of uncommon and subtle C−H bond changes induced by noncovalent interactions validates a long-standing proposal, the ability of a tetrel bond to carbon atom to lengthen its attendant C−H bonds.
Research Details
- The study was enabled by the effective separation of two chiral Zn complexes and the high-resolution neutron diffraction data from TOPAZ.
- The accurate determination of the hydrogen positions were used to affirm the nature of the interactions based on DFT and related calculations.
“Unusual Changes of C–H Bond Lengths in Chiral Zinc Complexes Inducted by Noncovalent Interactions”
Elakkat V., Tessema E., Lin C.H., Wang X.P., Chang H.C., Zheng Y.N., Huang Y.C., Gurumallappa G., Zhang Z.Y., Chan K.L., Asti H.R., Francisco J.S., and Lu N.,
Angewandte Chemie International Edition, 62, e202215438 (2023). DOI: doi.org/10.1002/anie.202215438




