by Leo Williams
Question: What do you get when you take two surfaces roughly the size of a celery seed and crush them together with a load of 15 tons?
Answer: You get pressures approaching those inside planets, allowing you to distort nearly any material beyond recognition.
Researchers with Oak Ridge National Laboratory's (ORNL's) Spallation Neutron Source (SNS) have developed technology to squeeze materials with a million times the pressure of Earth's atmosphere while studying them with neutrons. When they bombard these materials with neutrons, the materials provide an unprecedented picture of the changing nature of matter under extreme pressure.
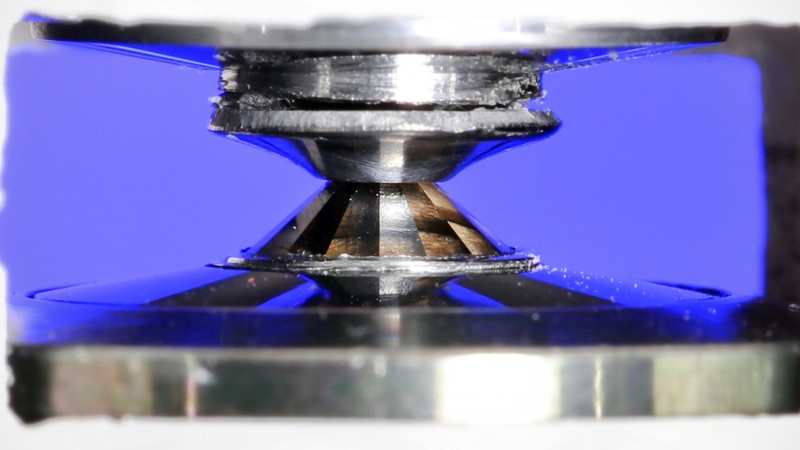
The technology is known as a diamond anvil cell. It uses diamonds whose tips have been polished off to create flat surfaces, then presses a sample between those surfaces with immense force to create an extreme experimental environment. Diamond anvil cells have been used in other types of experiments -- for X-ray scattering at synchrotrons and for optical spectroscopy techniques, for instance -- but bringing them into the realm of neutron science creates a unique opportunity, and an immense challenge.
The opportunity is that neutron scattering can examine light elements such as hydrogen and silicon in a way that no other method can. At the SNS SNAP instrument (SNAP stands for Spallation Neutrons and Pressure Diffractometer), scientists have been exploring a variety of scientific questions. For instance, research into the complex behavior of ice under extreme pressure may give us an idea of what's going on inside our solar system's gas giants, while explorations into the effect of hydrogen on materials deposited in a thin film may clear away impediments to more advanced electronics manufacturing.
Larger samples
"The exciting thing about pressure is you can put in so much more energy than you can with temperature," said Bianca Haberl, a Weinberg Fellow at SNS, which is a DOE Office of Science User Facility. "That means you can change the atomic bonding so much more. There is no other way to change materials as drastically as you can with pressure."
The challenge of this approach, however, is that neutron scattering requires far larger samples than techniques such as X-ray scattering. While that celery seed may look tiny on the tip of your finger, it is enormous in the world of advanced scattering techniques.
"If you do optical benchtop or X-ray experiments, it's in the order of a few tenths of a millimeter," said Reinhard Boehler, who divides his time between ORNL and the Carnegie Institution of Washington in Washington, D.C. "With neutrons, we need sample sizes that are a few hundred times larger, because neutron fluxes are typically very low."
This is such an enormous challenge because larger surface areas require much more force to reach the same pressure. In fact, neutron scattering with diamond anvil cells would be impossible without two major technological advances: SNS itself, which delivers the most intense pulsed neutron beams in the world, and recent breakthroughs in the creation of large synthetic diamonds.
The project needed much larger diamonds -- in the neighborhood of 10 carats -- because smaller diamonds and their supports break under that kind of force. In addition, researchers needed single-crystal diamonds, because composites that are fused together from smaller diamonds interfere with neutrons.
As you might imagine, such large diamonds are expensive; you'd be hard pressed to find a 10-carat natural diamond for under $200,000. Add to that the fact that diamonds are definitely not forever when they're pressed with 15 tons, and it becomes clear that the cost of natural diamonds makes them prohibitive.
Boehler said the diamond breakthrough was a technique called chemical vapor deposition, which was optimized for diamond growth by his lab at Carnegie and licensed to private companies for manufacture. The researchers can get 10-carat diamonds created with this technique for about $7,000.
Squeezing ice
Research with the new technology so far has included the study of water -- Boehler's specialty -- and the effect of hydrogen on thin films -- Haberl's. Both exploit the unique strength of neutron science by focusing on hydrogen -- nature's lightest element, with only one proton.
Boehler, whose background is in geophysics, has been squeezing ice and hydrogen, in part to replicate the interiors of planets such as Neptune and Uranus. So far he has focused on hydrogen and water molecules -- two hydrogen atoms and one oxygen -- but he also has his eye on methane (four hydrogens and one carbon) and ammonia (three hydrogens and one nitrogen).
His experiments at SNS so far have focused on ice containing deuterium, a hydrogen isotope whose nuclei contain a neutron as well as a proton. As commonplace as water is, he explained, it is far from humdrum when exposed to high pressure, where even its freezing point becomes controversial.
"Even the melting of water at high pressure is very controversial," he said. "If you give that problem to five groups, they will give you five different answers, and the differences are not small."
Using ice with deuterium, Boehler and colleagues, Chris Tulk, Antonio Moreira dos Santos and Jamie Molaison from SNS, and Malcolm Guthrie formerly of the Carnegie Institution, have been able to put samples under a million atmospheres of pressure. While they are still in the process of analyzing results, theorists have suggested that water under high pressures behaves like a crystal such as rock salt, or like a metal.
"Theoretically, it's very difficult to handle, because of very different interatomic forces for oxygen or hydrogen or deuterium," he said.
That pesky hydrogen
Haberl, whose background is in materials science, has been using diamond anvils at SNS to study the effects of hydrogen contamination on deposited thin films, such as the silicon used in electronics.
By thin, we mean films a thousand times thinner than a human hair.
"Most semiconductors nowadays are deposited," she said, "because it's thin. I'm talking about 10 nanometers to 100 nanometers, and the thinner you can make it and have it still work, the less material you need."
The problem with these deposited films is that they invariably end up containing hydrogen. Standard, hydrogen-free silicon that has been exposed to high pressure shows exciting behaviors and new structures, even after the pressure is removed. These behaviors may lead to valuable new technologies. The cheaper deposited silicon -- with hydrogen -- does not show the same behavior, and the same useful new structures cannot be synthesized.
"Every time you deposit something, you have to make a vacuum, which is never perfect," she explained. "In addition, you often start off with materials that contain hydrogen, so in the end your deposited film may contain up to 10 percent hydrogen."
By putting the deposited films under high pressure, Haberl said, she and her colleagues hope to make these thin films more useful. To understand why these deposited films with hydrogen do not result in these useful new structures, they need neutron scattering, simply because the hydrogen is invisible to X-rays.
Haberl noted that they are also interested in materials other than silicon -- for example extremely hard carbon films, germanium films that could replace silicon in semiconductors, or other so-called compound semiconductors
Looking forward, high-pressure research at SNS will expand both to new scientific areas and to other instruments in the facility, predominantly the VISION instrument. Research proposals coming into the facility include studies of pure carbon, hydrogen sulfide (a very promising superconductor), and even heavy elements such as actinides (a group that includes uranium and plutonium).
Boehler said the new technology, coupled with the ability of SNS to exploit neutrons in a variety of ways, will open up exciting new areas of knowledge.
"Now, with the new anvil technology, we have a big window of new opportunities."