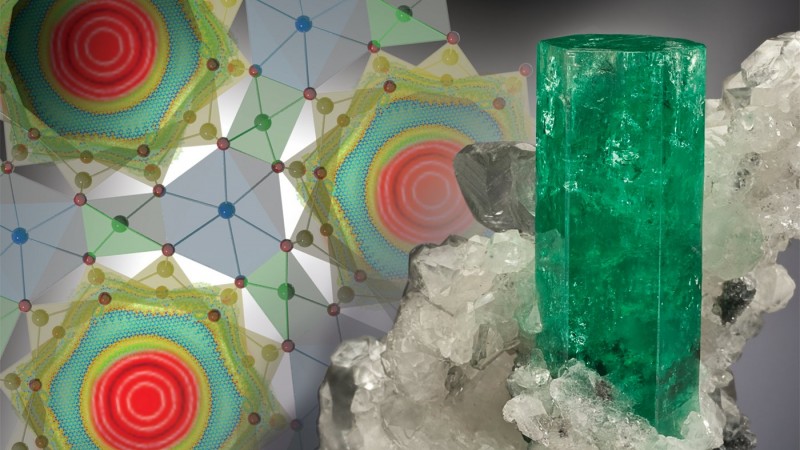
OAK RIDGE, Tenn., April 22, 2016 – Neutron scattering and computational modeling have revealed unique and unexpected behavior of water molecules under extreme confinement that is unmatched by any known gas, liquid or solid states.
In a paper published in Physical Review Letters, researchers at the Department of Energy’s Oak Ridge National Laboratory describe a new tunneling state of water molecules confined in hexagonal ultra-small channels – 5 angstrom across – of the mineral beryl. An angstrom is 1/10-billionth of a meter, and individual atoms are typically about 1 angstrom in diameter.
The discovery, made possible with experiments at ORNL’s Spallation Neutron Source and the Rutherford Appleton Laboratory in the United Kingdom, demonstrates features of water under ultra confinement in rocks, soil and cell walls, which scientists predict will be of interest across many disciplines.
“At low temperatures, this tunneling water exhibits quantum motion through the separating potential walls, which is forbidden in the classical world,” said lead author Alexander Kolesnikov of ORNL’s Chemical and Engineering Materials Division. “This means that the oxygen and hydrogen atoms of the water molecule are ‘delocalized’ and therefore simultaneously present in all six symmetrically equivalent positions in the channel at the same time. It’s one of those phenomena that only occur in quantum mechanics and has no parallel in our everyday experience.”
The existence of the tunneling state of water shown in ORNL’s study should help scientists better describe the thermodynamic properties and behavior of water in highly confined environments such as water diffusion and transport in the channels of cell membranes, in carbon nanotubes and along grain boundaries and at mineral interfaces in a host of geological environments. Read the full story.